Leave Your Message
In the realm of laboratory operations, the significance of a reliable Lab Water Purification System cannot be overstated. Water purity is crucial, as contaminants can compromise experimental results, affect analytical accuracy, and lead to erroneous conclusions. According to a recent report by the International Society for Analytical Chemistry, over 70% of laboratory errors are linked to water quality issues, highlighting the importance of investing in an appropriate purification system.
Selecting the right Lab Water Purification System involves a comprehensive understanding of specific laboratory needs and the types of impurities present in the source water. Industry standards indicate that systems must be capable of producing water with resistivity of 18.2 MΩ·cm at 25°C to meet the stringent requirements of high-performance applications. Additionally, the global market for laboratory water purification systems is projected to reach USD 3.5 billion by 2026, as researchers and institutions increasingly recognize the need for high-quality water in maintaining the integrity and reproducibility of scientific investigations.
Thus, choosing the suitable Lab Water Purification System goes beyond mere functionality; it requires an evaluation of different technologies, such as reverse osmosis, distillation, or deionization, to ensure that the system aligns well with specific laboratory applications. This article aims to provide a detailed guide to help researchers and laboratory managers navigate the complexities of selecting the ideal water purification solution for their distinct needs.

When selecting the right lab water purification system, it's essential to understand the various types available to meet your specific needs. The most common systems include reverse osmosis (RO), deionization (DI), and distillation. Each technology offers unique benefits: RO effectively removes dissolved solids, while DI targets ionic contaminants, and distillation eliminates microbes and non-volatile compounds.
Tips: When deciding which system is best for you, consider the water quality you require for your applications. For instance, if your work involves sensitive experiments, a combination of RO and DI may offer the purity you need. Additionally, evaluate the volume of water you will use; some systems are designed for high-throughput labs, while others are more suited for smaller-scale needs.
Another option worth considering is the ultrafiltration (UF) system, which uses a membrane to filter out larger particles and bacteria. This can serve as a pre-treatment step for more advanced purification processes. When looking for a purification system, assess the maintenance and operation costs, as these factors can significantly impact the long-term efficiency and practicality of the system you choose.
This chart illustrates the different types of lab water purification systems available in the market based on their common usage in laboratories. The categories include Distillation, Reverse Osmosis, Deionization, and Filtration, showcasing their relative prevalence in usage.
When selecting a lab water purification system, it's crucial to consider several key factors that can significantly impact the quality of your research. First, understand the specific requirements of your applications. Different processes, such as molecular biology or analytical chemistry, may need varying levels of purity. For instance, while some applications only require basic deionization, others may necessitate ultrapure water with extremely low levels of contaminants.
Another important factor is the capacity of the system. Assess your laboratory's water consumption to ensure that the chosen purification system can meet your daily needs without frequent interruptions. It's also wise to consider the system's efficiency and maintenance requirements. Systems that require less frequent maintenance can save you time and reduce downtime, allowing your lab to operate smoothly.
Tip: Before making a purchase, consult with your team or other users to get insights about their experiences with specific systems and types of technology. This can help you weigh the pros and cons effectively.
Finally, think about your budget. While it's tempting to opt for the cheapest solution, a more expensive system might offer better longevity and cost-efficiency in the long run. Evaluate the total cost of ownership, factoring in replacement filters, energy consumption, and maintenance costs, to make an informed decision.
Tip: Don't hesitate to reach out to suppliers for detailed specifications and case studies that reflect upon the system's performance under conditions similar to your laboratory's. This will ensure that you choose a purification system that truly meets your needs.
When evaluating your lab's water quality requirements, the first step is to identify the specific purification needs based on the applications and experiments to be conducted. Different tasks may require varying levels of water purity. For instance, analytical chemistry may demand ultrapure water with low ion content, while biological applications could necessitate water that is free from contaminants that affect cell cultures. Understanding these specific needs is essential to ensure that the water purification system you choose will deliver the quality required to produce accurate and reliable results.
Additionally, assessing the initial water quality is crucial. Conduct a thorough analysis of your source water's physical and chemical properties, including parameters such as conductivity, TOC (Total Organic Carbon), and microbial content. This assessment will aid in determining the necessary pre-treatment processes and filtration methods to employ before purification. By aligning your lab's unique requirements with the proper purification solutions, you will ensure not only the integrity of your experiments but also the efficient operation of the purification system itself.
When selecting a lab water purification system, it’s essential to understand the various purification technologies available and their respective applications. Common methods include reverse osmosis, distillation, deionization, and filtration. Reverse osmosis is highly effective in removing dissolved solids and contaminants, making it suitable for applications requiring high-purity water. Distillation, on the other hand, is ideal for generating ultrapure water by boiling and condensing, removing inorganic and organic impurities. Deionization is specifically useful for applications requiring low ionic content, such as in laboratory experiments and certain pharmaceutical processes.
Tips: When evaluating purification technologies, always consider the specific requirements of your applications, such as the desired water quality, volume, and source water conditions. Additionally, take into account the maintenance needs and operational costs associated with different systems to ensure sustainability in the long run.
Filtration systems can also play a crucial role, particularly for removing particulate matter and larger contaminants. They are often used as a pre-treatment step before other purification methods. Understanding the right combination of these technologies can optimize your lab's water purification process, ensuring you achieve the purity levels necessary for accurate research and experimentation.
Tips: Assess your current and future water needs, and consult with experts to determine the best combination of purification technologies that align with your lab’s goals.
| Purification Technology | Description | Advantages | Applications |
|---|---|---|---|
| Reverse Osmosis (RO) | Uses a semipermeable membrane to remove impurities from water. | High purification efficiency; removes dissolved solids. | Laboratories, pharmaceuticals, and electronics. |
| Ultra Filtration (UF) | Employs membrane technology to separate particles based on size. | Effective for removing bacteria and large molecules. | Biotechnology and food industry. |
| Distillation | Involves boiling water to produce steam, then condensing it back into liquid. | Removes a wide range of contaminants, including salts. | Medical labs and research facilities. |
| Deionization (DI) | Removes ions from water using ion exchange resins. | Produces high purity water; effective for specific ion removal. | Semiconductor processing and gold plating. |
| UV Filtration | Uses UV light to disinfect water by destroying microorganisms. | Chemical-free disinfection; low operational costs. | Laboratories and water purification systems. |
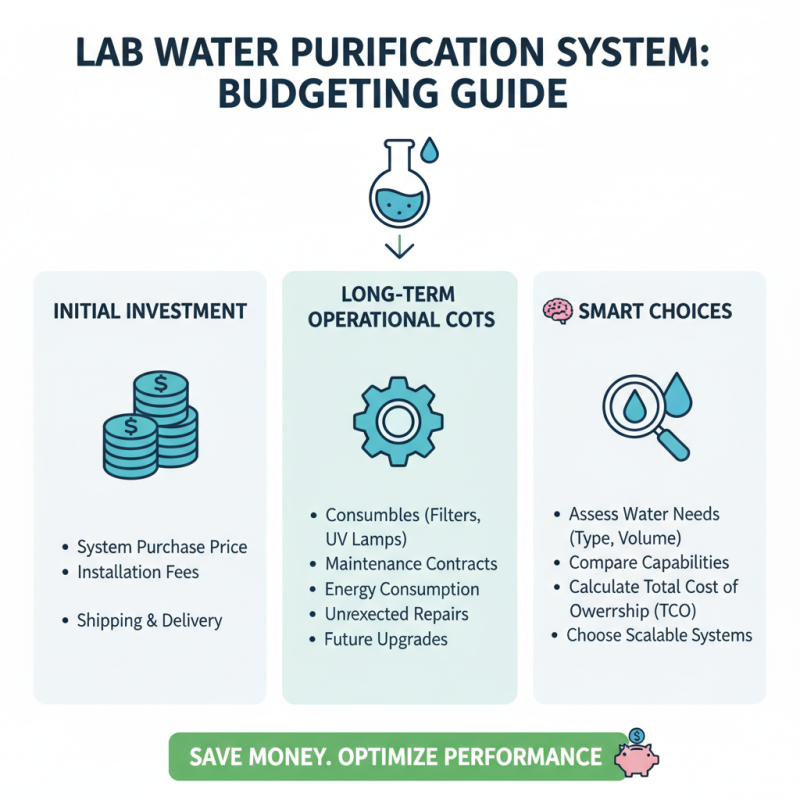
When budgeting for a lab water purification system, it's essential to consider both the initial investment and the long-term operational costs. Choosing a system that fits your specific needs can prevent overspending. Start by assessing the type and volume of water your lab requires. Systems can vary significantly in cost, depending on their capabilities. Make sure to account for installation fees, maintenance contracts, and potential upgrades needed in the future.
Tips: Always conduct a cost-benefit analysis to weigh the advantages of a more expensive, high-performance system against a less costly, more basic model. Remember to factor in the cost of consumables, such as filters and replacement membranes, as these can add up over time.
Moreover, seeking quotes from multiple suppliers can help you identify competitive pricing. As some systems have hidden costs, be vigilant and ask detailed questions to understand the total financial commitment. Consider exploring financing options or leasing programs if upfront costs are a concern. Prioritize your lab's specific water quality requirements while also being mindful of your budget constraints to ensure the best decision is made for both performance and affordability.






